The European Medicines Agency (EMA) has made it possible for Russian-made Sputnik V to be registered in the Union. Scientific consultations on this medicine are over.
Sputnik Azerbaijan reported this on February 9, 2021, citing RIA Novosti.
Fakt Yoxla examined whether the news was true.
The European Medicines Agency (EMA) is the body that conducts scientific assessments of applications for the sale of medicines within the European Union.
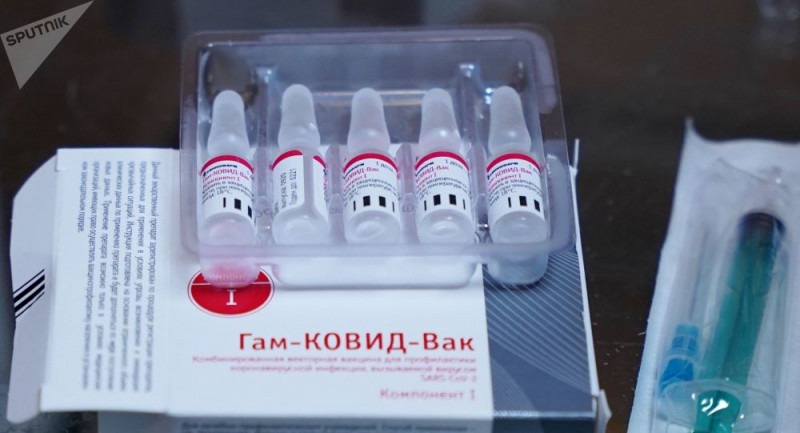
EMA issued a statement on the registration of the Sputnik V vaccine on February 10, 2021. Contrary to the allegations, the agency has not yet received an application for periodic review and marketing permission for the Sputnik V vaccine (Gam-COVID-Vac) developed by the Gamaleya National Research Center of Epidemiology and Microbiology in Russia.
The developers of the vaccine received scientific advice from the EMA, which, in turn, informed them about the latest regulations. According to the agency's transparency policy, a list of medicines and vaccines for which scientific advice is obtained is published. The list also shows the phase of the medicine. The Sputnik V vaccine is also included in the list for this reason.
EMA is in constant contact with the company to follow the next steps. Such cases occur rarely. Based on the situation created by the pandemic, dialogues are held so that the agency can evaluate the vaccine as soon as it is ready.
A list of vaccines currently under evaluation is also available on the agency's website. Sputnik V is not on this list. This means that the agency has not evaluated the vaccine, and in general, the agency has not received such an application.
Fakt Yoxla concludes that the news is FAKE NEWS.